OVERVIEW
This review begins with the relationship between sympathetic drive and heart rate at each step along the continuum of cardiovascular disease, which has been adapted from the work of Drs Vasan and Levy1 (Fig. 1). As shown, heightened sympathetic drive and faster heart rates are linked with endothelial dysfunction, markers of systemic inflammation, incident hypertension and diabetes, left ventricular hypertrophy, vascular remodeling, myocardial infarction, systolic dysfunction, heart failure, and death. Left ventricular hypertrophy, myocardial infarction, and heart failure are also associated with an increased risk of sudden death. Increased sympathetic drive and faster heart rate contribute to sudden death.
Faster heart rates beginning in the upper seventies (beats per minute), which often receive little to no clinical attention, are associated with increased risk for hypertension, diabetes, cardiovascular events, and sudden death. Moreover, increased sympathetic drive and faster heart rates participate in the systolic dysfunction that often follows a myocardial infarction including progression to heart failure. Among patients with heart failure, increased sympathetic drive and faster heart rates are linked with morality and sudden death. While not shown, β-adrenoceptor blocking agents can interrupt this continuum at several points in the progression with stronger evidence for secondary than primary prevention of cardiovascular disease.
In the beginning, increased sympathetic drive and faster heart rates contribute to cardiovascular risk factors including hypertension and diabetes. The genesis or starting point for elucidating an important role for increased sympathetic drive and faster heart rates to the cardiovascular continuum is perhaps the most important as one could posit that the later changes simply reflect the response to the underlying pathophysiological changes. Consequently, this section on the relationship of increased sympathetic drive and faster heart rates in the initial stages of the cardiovascular continuum are provided in more details than the middle and late phases of the continuum which follow.
The antecedents of cardiovascular disease typically begin with few if any classical clinical symptoms. These antecedents include endothelial dysfunction, a relative resting tachycardia, modest nonhypertensive elevations of blood pressure, hyperinsulinemia, insulin resistance, a complex dyslipidemia, and markers of systemic inflammation (Fig. 1). The complex dyslipidemia associated with insulin resistance is typically characterized by modest elevations of triglycerides and reductions of high-density lipoprotein (HDL) cholesterol, and denser low-density lipoprotein (LDL) cholesterol with heightened sensitivity to oxidative changes, which renders it more atherogenic.2 Pathologic changes in the lipoprotein profile can occur without clinically elevated total or LDL-cholesterol, and requires heightened clinical awareness for detection. Left unchecked, these antecedents can progress to clinical hypertension and diabetes.
Fig. 1
The cardiovascular continuum viewed from a neurogenic perspective.1 Increased SNS activity and faster HR are associated with endothelial dysfunction, inflammation, incident hypertension and diabetes, LVH, vascular remodeling, and MI; LVH and MI are linked with sudden death. Increased SNS activity and faster HR participate in the systolic dysfunction that often follows an MI including progression to HF; HF, in turn, substantially increases mortality including sudden death, which are associated with increased SNS drive and faster HR. While not shown, β-blockers can interrupt this continuum with stronger evidence for secondary than primary prevention of cardiovascular disease. LVH: Left ventricular hypertrophy; MI: Myocardial infarction

A substantial body of literature links heightened sympathetic drive and a relative tachycardia to the aforementioned antecedents of hypertension and dysglycemia, which include endothelial dysfunction, systemic inflammation, and insulin resistance.3-7 Graph 1, adapted from prior publications, displays the link between a relative resting tachycardia and subsequent development of clinical hypertension and diabetes.3,4
Progression from Sympathetic Overdrive and Faster Heart Rates to Hypertension
Harburg et al8 reported that male college students with sustained elevations of blood pressure at screening and home described themselves as motivated to obtain social contacts, but in a “sensitive” and “anxious” manner. Young men who had an elevated screening and subsequent office blood pressure yielded more frequently in an argument and subsequently change their private opinions to agree with partners who had lower blood pressures. A subsequent study found that among undergraduate men with elevated screening blood pressures, the subset with elevated blood pressure values at home reported greater intensity of anger and suppressed their anger to a greater extent than those with normal home blood pressure.9
Anger, Increased Sympathetic and Reduced Parasympathetic Tone
Marci et al10 studied emotional recall and sympathetic and parasympathetic activity. Of emotions studied, only anger was linked with increased sympathetic and decreased parasympathetic activity.
Role of Sympathetic Activation and Parasympathetic Inhibition in the Hyperkinetic Hemodynamic Profile of Borderline Hypertension
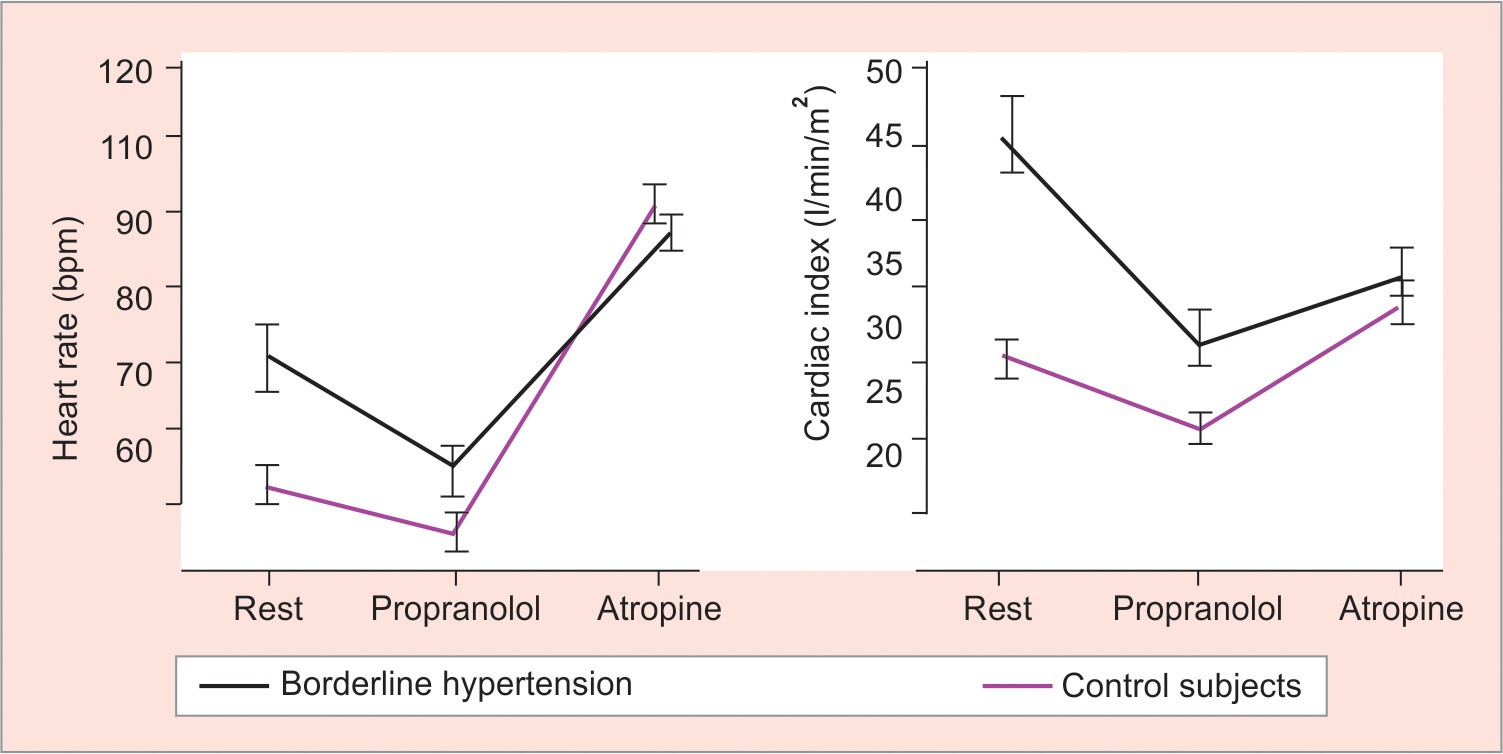
Hyperkinetic borderline hypertensives had higher values than healthy normal controls for cardiac index and heart rate (Graph 1). β-adrenoceptor blockade led to a larger fall of cardiac index and heart rate among individuals with hyperkinetic borderline hypertension than controls, yet values remained higher in the hyperkinetic group. With atropine to block cardiac vagal tone, cardiac output and heart rate increased less in subjects with hyperkinetic borderline hypertension than in normal controls and were no longer different from normal controls.11 These data suggest that increased sympathetic and reduced parasympathetic tone contribute to hyperkinetic pre-hypertension.
Graph 1
Sympathovagal imbalance plays a major role in the cardiac changes characteristic of hyperkinetic prehypertension.11 Baseline heart rate and cardiac index are higher in hyperkinetic prehypertensives than age- and sex-matched normal controls. After β-adrenoceptor blockade with propranolol, heart rate and cardiac index decline more in the hyperkinetic prehypertensive than normal group, yet values remain higher in the former. With addition of the muscarinic receptor antagonist atropine to eliminate cardiac vagal tone, heart rate and cardiac index increase less in the hyperkinetic than in the control group. At that point, group difference in heart rate and cardiac index was no longer significant
Transition from Hyperkinetic Borderline Hypertension to Normokinetic Borderline and Established Essential Hypertension
It is important to note that studies employing muscle sympathetic nerve activity and norepinephrine turnover documented sympathetic overactivity in a large proportion of adults with essential hypertension including those who are obese.12-14 Faster heart rates, even within the range of 60 to 100 that are considered normal, are linked with incident hypertension (Graph 2).3 Many hyperkinetic subjects appear to develop classical established hypertension over time,15 yet the hyperkinetic state is much less common in adults with hypertension. Thus, a transition almost certainly occurs from the hyperkinetic borderline hypertension to normokinetic, high-resistance hypertension.15
Graphs 2A and B
(A)Transient tachycardia and transient hypertension increase risk for future (upper),3 while relative resting tachycardia raises risk for diabetes (lower).4 Upper panel: The relationship between transient tachycardia [(tTachy) ≥100 beats/min], transient hypertension [(tHTN) ≥150/≥90 mm Hg] on risk of future hypertension is shown. Both transient tachycardia and transient hypertension approximately double the risk for future hypertension, whereas the combination of both trebles risk. Adapted from data in reference Levy et al.3 (B) Lower panel: Resting heart rate and the adjusted odds ratio for future diagnosis of diabetes mellitus (Dx DM) and death (DM Death) are shown. Heart rate and risk of diabetes were similar in younger (<50 years) and older (≥50 years) patients at baseline, whereas the risk of death was observed only in those <50 years at baseline (odds ratio death shown only for <50 years group). Adapted from data in Carnethon et al4

Julius et al16 conducted studies supporting a transition from neurogenic hyperkinetic to neurogenic normokinetic prehypertension and established hypertension. Heart rate was elevated less in subjects with prehypertension and normal cardiac output than in hyperkinetic prehypertensives. After cardiac autonomic blockade with propranolol and atropine, cardiac output in “normokinetic” prehypertensive group was lower than in normal controls. Heart rate responses to isoproterenol were less in the normokinetic prehypertensives than normal controls and likely reflect β-adrenoceptor downregulation in response to persistent elevations of sympathetic drive. Following cardiac autonomic blockade, the mild blood pressure elevation in the normokinetic prehypertensives was sustained by increased vascular resistance, a key pathophysiological feature in human essential hypertension.
Esler et al17,18 then showed that the elevated blood pressure in prehypertensive and hypertensive individuals with high plasma renin activity values was neurogenic. The effects of heightened sympathetic drive include high plasma renin activity via activation of β-adrenoceptors on renin secreting cells of the juxtaglomerular apparatus. Following total autonomic blockade with propranolol (β-adrenoceptor antagonist, atropine vagolytic, muscarinic receptor antagonist) and phentolamine (α-adrenoceptor antagonist), blood pressure values declined to normal in the high-renin group, while remaining elevated in the low and middle renin groups. Subsequent studies with regional infusions of phentolamine in the human forearm confirmed the presence of increased vascular α-adrenergic tone at an early stage of hypertension and in obese prehypertensives.19,20
Mechanisms underlying the Hemodynamic Transition from Hyperkinetic Borderline Hypertension to Normokinetic Hypertension
Decreased β-adrenergic sensitivity, cardiovascular remodeling, increased vascular α-adrenergic tone, and resetting of renal pressure-natriuresis.
Cardiac changes. Sustained increases in cardiac sympathetic drive lead to decreased chronotropic and inotropic and probably to the lusitropic responses to. β1-adrenoceptor activation.16,21-23 Julius and Majahalme22 proposed that the combination of downregulation of cardiac β1-adrenergic receptors with decreased chronotropic and inotropic responses to sympathetic drive together with decreased cardiac compliance and stroke volume contribute to the normalization of cardiac output in established hypertension.
Vascular changes. Concurrent structural vascular remodeling secondary to elevated blood pressure and sympathetic drive support the progressive rise in vascular resistance.24,25 Folkow26 was a pioneer in showing how an increased wall:lumen ratio contributes to hypertension by raising vascular resistance and nonspecifically amplifying resistance responses to various vasoconstrictors. Subsequent studies by the Ann Arbor group in relatively young subjects with Stage 1 hypertension compared with demographically and weight-matched normotensive controls were consistent with vascular remodeling as a nonspecific amplifier of arterial resistance in response to different vasoconstrictors.19,27 Vasodilator responses in the forearm to regional phentolamine were also greater in Stage 1 hypertension than in controls, indicating greater vascular α-adrenoceptor-mediated tone.19
Resetting of the Renal Pressure/Natriuresis Relationship
Guyton and Coleman28 demonstrated that the kidney is the predominant long-term regulator of arterial blood pressure.29 Unless the renal pressure natriuresis relationship is set to a higher level, then hypertension cannot be sustained. Similarly, lifestyle and/or pharmacotherapy that produce a sustained reduction in blood pressure must lower the pressure set point for renal pressure/natriuresis. The increased sympathetic drive can reset the renal pressure/natriuresis threshold upward by increasing renin secretion and the subsequent cascade to angiotensin II and aldosterone and by directly activating α1-adrenoceptors on the renal tubules.30,31 In humans, acute stress induced significant renal sodium retention that correlated directly with the rise of heart rate, with the clear implication that both were neurogenically mediated.32 Moreover, long-term studies in animals document that stress can induce a sustained resetting of the renal pressure/natriuresis relationship to higher pressure.33
Sympathetic Activation and the Cardiometabolic Syndrome
In a community, population-based sample of young adults, Julius et al34 demonstrated that heart rate, a marker of sympathetic activation, correlated with several cardiometabolic syndrome features, including insulin, glucose, triglycerides, and HDL-cholesterol in young adults at an early phase of hypertension.7 The relationship between heart rate and hyperinsulinemia was especially strong. The hyperinsulinemia most likely represents a compensatory response to insulin resistance given the inverse relationship with HDL-cholesterol and direct link with hypertriglyceridemia.
Sympathetic Drive in Hypertension and Diabetes
Direct sympathetic nerve recordings in patients with hypertension alone, diabetes alone, and both hypertension and diabetes together. Compared with normal control, hypertension and diabetes are each associated with increased sympathetic neural activity (Graph 3).35 The combination of hypertension and diabetes is linked with greater sympathetic nerve activity than either of the component conditions alone. Evidence presented subsequently suggests this heightened sympathetic activity contributes to the insulin resistance in patients with diabetes and/or hypertension as well as the heightened risk linked with diabetes and hypertension alone and the amplified risk when both are present.
Graph 3
Sympathetic nerve activity in hypertension and diabetes alone and combined.36 Muscle sympathetic activity in bursts or impulses/100 heart beats is shown for normotensive (NT) controls, patients with diabetes (DM) and essential hypertension (EHT) alone, and the combination (EHT + DM). Sympathetic nerve activity is increased in both DM and EHT compared with NT controls and greater in EHT + DM than with DM or EHT separately

Skeletal muscle is a key target organ for insulin action.36 Resistance to insulin-mediated glucose disposal, a dominant feature of the cardiometabolic syndrome, is exacerbated by increased vascular α-adrenergic tone,37,38 a key feature in neurogenic hypertension.17,18 Jamerson et al37,38 demonstrated that insulin-mediated glucose disposal in the human forearm was acutely reduced in response to thigh-cuff inflation. This stimulus pools blood in the lower extremities, thereby unloading cardiopulmonary mechanoreceptors and inducing reflex neurogenic forearm vasoconstriction. With similar reductions in forearm blood flow, reflex neurogenic vasoconstriction induced more forearm insulin resistance than an intraarterial norepinephrine infusion. Thus, reflex neurogenic vasoconstriction reduced glucose utilization by mechanisms other than or in addition to reduced blood flow.
Jamerson et al37,38 cited evidence that reflex neurogenic vasoconstriction reduces the number of open capillaries in skeletal muscle. Capillary density in skeletal muscle is a major determinant of insulin-mediated glucose disposal.39 Their experimental data suggested that neurogenically-mediated vasoconstriction,37,38 observed in high-renin patients with borderline and established essential hypertension,17,18 significantly diminishes insulin-mediated glucose disposal in skeletal muscle. This notion is consistent with studies showing that selective α1-adrenoceptor antagonists improve insulin-mediated glucose disposal in hypertensive patients to a greater extent than renin/angiotensin system blockers.40,41 Subjects in the Tecumseh Blood Pressure Study who had higher hematocrits, which likely reflect a neurogenically mediated reduction in plasma volume, also had greater values for plasma norepinephrine and renin values than healthy controls.42,43
MID-PHASE OF THE CARDIOVASCULAR CONTINUUM AND THE ROLE OF INCREASED SYMPATHETIC DRIVE AND FASTER HEART RATES
Overview
Obesity, hypertension, and diabetes are associated with left ventricular hypertrophy. These three risk factors together with the dyslipidemia, inflammation, and thrombogenic diathesis are associated with atherosclerosis and related clinical events including acute myocardial infarction and stroke. Left ventricular hypertrophy and acute myocardial infarction, in turn, are associated with sudden death.
Sympathetic Drive, Left Ventricular Hypertrophy in Hypertension, and Sudden Death
In a study of hypertensive patients, the subset with greater muscle sympathetic nerve activity had greater left ventricular mass than those with lower levels of muscle sympathetic nerve activity.44 It is likely that difference in sympathetic nerve activity contributed to the left ventricular hypertrophy as arterial blood pressures were similar in the two groups. In adults with left ventricular hypertrophy, the incidence of sudden death increases from ~0.25% annually to 1.8% annually as wall thickness increases from 16 to 19 mm to >30 mm.45 In the Framingham Heart Study, increased left ventricular mass and left ventricular hypertrophy were associated with a significantly increased risk for sudden death.46 The absolute risk for sudden death with cardiac hypertrophy was greater in men than women in Framingham.
Heart Rate, Cardiovascular Events, and Mortality
Among men in the Paris Prospective study, the subset with resting heart rates of 65 to 70 had roughly a 50% increase in the relative risk of death from myocardial infarction, cardiovascular disease, and all-causes as well as a doubling in risk of sudden death compared with men with resting heart rates <60 beats/minute (Graph 4).47 Among men with resting heart rates above 75 beats men, death from myocardial infarction, cardiovascular disease and all-cause were double and sudden death is four-fold higher than in men with resting heart rates <60 beats/minute. Similar results were observed among men in the Framingham Heart Study with death rates from myocardial infarction, cardiovascular disease, and all-cause substantially higher in the groups with heart rates 75 to 84 and >84 beats/minute than in those with heart rate <65 beats/minute.48 These studies confirm that a relative resting tachycardia, which often does not receive clinical attention, is not benign but associated with clinically important excess risk for cardiovascular and all-cause mortality.
Graph 4
Resting heart rate and morality among men in the Paris Prospective Study.48 A total of 7,079 men ages 42 to 53 years were followed for 23 years in the Paris Prospective Study.48 The relative risks for total, cardiovascular, and sudden death as well as fatal myocardial infarction are shown with quintile one as the reference group. As indicated, relatively modest elevations of resting heart rates, which are often considered normal, were associated with an increased relative risk of death, particularly sudden death

Heart Rate and Outcomes in Adults with Treated Hypertension at High Risk for Cardiovascular Events
In the Valsartan, Amlodipine Long-term Use Evaluation (VALUE) study, individuals with blood pressure controlled to <140/<90 but heart rates in the upper quintile, with resting heart rate of ~80 beats/minute and higher, had a 53% higher incidence of the primary outcome of nonfatal heart disease and stroke and cardiovascular death than those with controlled blood pressure and heart rate in the lower four quintiles.49 Patients with heart rates in the upper quintile and controlled hypertension had a minimal reduction in events compared with those with heart rates in the upper quintile and uncontrolled hypertension.
Heart rate is a relevant predictor of cardiovascular outcomes in adults who are treated for hypertension. However, the management of heart rate in hypertensive patients for the primary prevention of cardiovascular disease remains controversial with a limited evidence base to guide clinicians.50 In contrast, evidence for β-blockers in secondary prevention for adults with coronary heart disease or chronic heart failure and reduced ejection fraction (HFrEF) is solid.
LATE PHASE OF THE CARDIOVASCULAR CONTINUUM: HFrEF AND DEATH
Faster heart rates and increased sympathetic drive measured by plasma norepinephrine and muscle sympathetic nerve activity are associated with poor outcomes in patients in the latter phase of the cardiovascular continuum. The poor outcomes include the development of HFrEF and related hospitalization and death.51,52 The association of faster heart rates with adverse outcomes is clearly evident among heart failure with preserved EF patients with sinus rhythm but less apparent in those with atrial fibrillation.53 Moreover, the benefits of β-blockers at this late stage for reducing hospital admissions and death have been linked to heart rate reduction.53,54 Ivabradine, which lowers heart rate through effects on the funny channels in sinoatrial pacemaker cells, is also proven to reduce hospital admissions in HFrEF patients.55
SUMMARY
We have examined evidence that increased sympathetic drive and/or faster heart rates contribute to the pathogenesis and complications of the cardiovascular continuum. There is substantial evidence for the SNS and faster heart rates as primary factors in the early phase of the continuum. Behavioral factors and environmental stressors including access to excess, especially when it results in abdominal-visceral obesity, are key factors underlying long-term sympathetic activation and multiple features of the cardiometabolic syndrome. Lifestyle and public health measures have the potential to significantly reduce the prevalence of cardiometabolic risk factors and to slow the progression of the cardiovascular continuum. While there are data supporting sympatholytic therapy for primary prevention of cardiovascular diseases, especially in younger and middle-aged men, the evidence for beneficial effects of β-blockers is more clearly established for secondary prevention. Further research to identify effective pharmacological tools for the primary prevention of cardiometabolic risk factors and related clinical complications is a priority given the growing global disease burden.